Executive Summary
The Life Sciences industry is constantly evolving, driven by innovations, market dynamics, and regulatory changes. One critical aspect that often goes overlooked is the management of Loss of Exclusivity (LoE). LoE represents a pivotal point in a product’s lifecycle when market exclusivity ends, opening the door to generic competition. Unfortunately, many companies fail to proactively plan and manage their LoE transitions, leaving them vulnerable to immense revenue erosion and market share loss. This does not only account for big pharma, which have a wide-range of products in their portfolio, and many have already faced the patent cliff, but may be even more critical for Emerging and Biotech companies, who have not yet reached LoE for one of their products and hence, only limited to no experience.
This article explains in the beginning what “Loss of Exclusivity” (LoE) means and then explores the pressing need for Life Sciences companies to properly prepare and conduct their LoE management. After, it introduces a comprehensive suite of activities designed to empower Life Sciences companies to navigate this challenging phase strategically. By building insights to derive strategies, capabilities, and tools, companies can capitalize on their advantages while mitigating risks during the LoE period to maximize the value retention. The emphasis of this article is mainly for pharmaceutical manufacturs with a focus on markets and regulations outside the US.
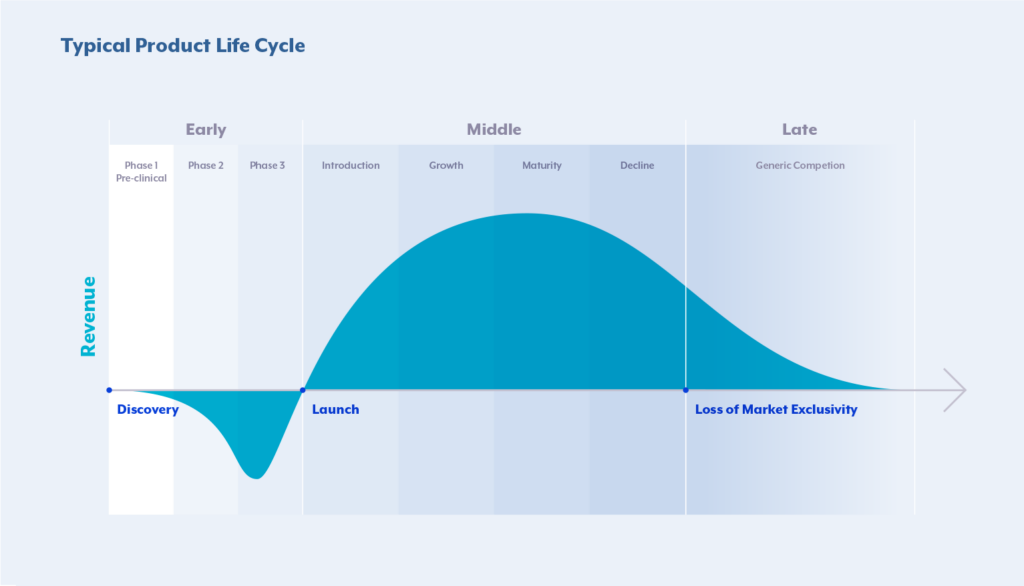
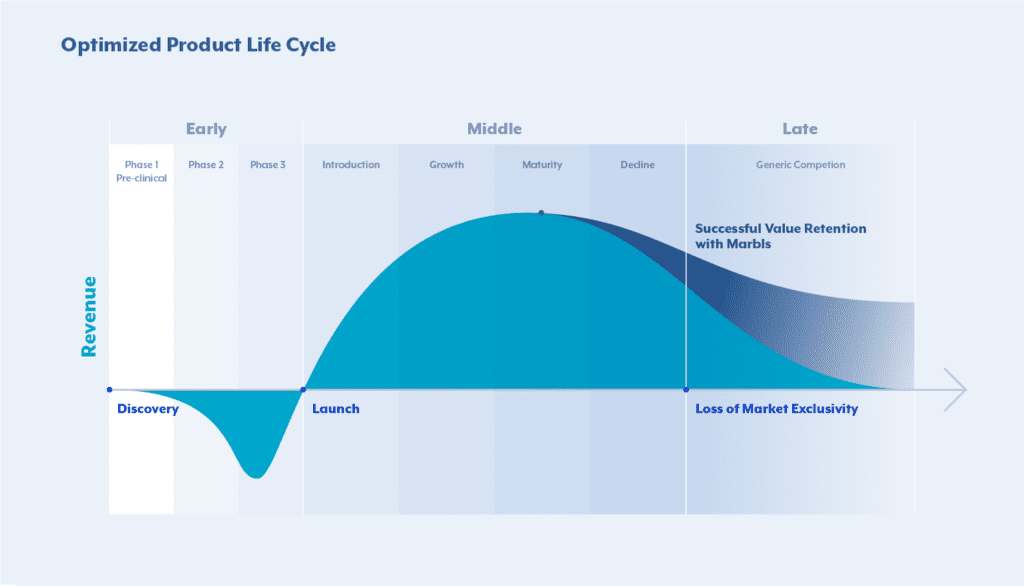
The Patent Cliff — the end of the Product Lifecycle given its Loss of Exclusvity (LoE)
“Loss of Exclusivity” (LoE) in the context of life sciences, particularly pharmaceuticals, refers to the point in time when a drug loses its patent protection or other forms of market exclusivity. Pharmaceutical companies typically receive patents for their new drugs, granting them exclusive rights to manufacture and sell the drug for a certain period, usually around 20 years from the filing date of the patent. Once the patent protection expires, other companies can
produce and market generic or biosimilar versions of the drug. In Europe, the regulation of the Generic and Biosimilar market access pathway is stated within the “Directive 2001/83/EC” and “Regulation (EC) No 726/2004” which aims to facilitate the approval and market access of these types of medicinal products, while ensuring their quality, safety, and efficacy. Both generic and biosimilar products play a crucial role in leading to increased access to more affordbale medications for patients, as well as in promoting competition in the pharmaceutical market, to achieve cost savings for healthcare systems while maintaining rigorous standards for quality and safety. The regulatory framework aims to strike a balance between encouraging the development of these products and ensuring that they meet the necessary standards for approval and post-marketing surveillance. Additionally, many governments have implemented national legislations or guidelines to promote the adoption of generics and biosimilar, such as the “Act for the Financial Stabilization of the German Statutory Health Insurance System” in Germany with increased mandatory price cuts, or the “Social Security Financing Act” in France with intensified substitution rules.
Therefore, loss of exclusivity (LoE) is a critical event for pharmaceutical companies because it results in increased competition, often leading to a significant reduction in the price of the medication. The entry of generic versions can impact the market share and revenue of the original drug’s manufacturer. Understanding the timeline of LoE is essential for both the original drug manufacturer and competitors interested in entering the market with generic alternatives. To counteract this, companies may not only employ various activities, such as developing new formulations, seeking additional indications, or introducing follow-up drugs with improved features, but also pro-actively plan their Commercial & Pricing Strategy, as well as develop organizational readiness by establishing robust capabilities, tools & governance.
The Imperative of Proper LoE Management
The Pitfall of Neglecting Proactive Planning
It is not uncommon for Life Sciences companies to focus their attention and resources primarily on the development of new products and the growth of existing in-market brands. Unfortunately, the vital process of LoE planning often takes a backseat. This neglect can have significant consequences as it leaves companies ill-prepared to address the challenges that come with LoE, including patent expirations and generic competition, which can create a blind spot that jeopardizes the company’s long-term stability, profitability and hence, sustainability.
Therefore, one of the key reasons why proactive planning is crucial stems from the highly competitive and regulated nature of the Life Sciences industry. With patents expiring and generic competitors entering the market, the landscape becomes increasingly volatile, making it essential for companies to anticipate and prepare for potential challenges well in advance. If not, the company risks to not only jeopardize revenue streams and market shares but also compromises the company’s ability to maintain a competitive edge in an ever-evolving industry. Moreover, the implications of overlooking proactive planning extend beyond financial setbacks. Without a robust strategy in place, Life Sciences companies may struggle to effectively communicate changes to stakeholders, including investors, employees, and regulatory authorities. This lack of preparation can erode trust and credibility, damaging the company’s reputation and potentially impacting future partnerships and collaborations. Additionally, a delayed response to LoE challenges can lead to over-rushed decision-making, resulting in suboptimal solutions that may further diminish the company’s market position and inhibit its ability to recover effectively.
Given the multifaceted nature of the Life Sciences industry, proactive planning should ideally commence at the early stages of product launch and more importantly, continue throughout the entire lifecycle of a product and become integral at a minimum two to three years prior to LoE. By integrating strategic planning into the initial launch phase, and seeing it through the product lifecycle, companies can not only anticipate potential obstacles but also incorporate contingencies into their business models. Furthermore, by fostering a culture of foresight and adaptability within the organization, companies can effectively stay ahead of market shifts and regulatory changes, ensuring a sustainable and resilient position in the industry, throughout peri-LoE and even long post-LoE.
The Challenge of Resource Allocation
Resource constraints and the urgency of new product launches often lead to underestimating the importance of LoE management. While the allure of new products is undeniable, insufficient resource allocation for LoE can create significant challenges. Companies must recognize the complex nature of post-patent expiry transitions and implement well-defined resource allocation strategies. Adequate allocation of financial, human, and technological resources ensures thorough market research, effective pricing strategies, and robust marketing campaigns, mitigating the impact of generic competition.
Navigating the Regulatory Landscape
The Life Sciences industry is heavily controlled, and regulatory changes can significantly impact LoE management. These regulatory changes can have a direct consequence on product exclusivity, market entry, and competitive positioning, and necessitate a thorough understanding. Also, the regulatory landscape has various nuances across different regions, and country-by-country, with specific requirements and timelines in each target market. Moreover, establishing robust monitoring mechanisms, engaging in proactive dialogue with regulatory authorities, and participating in industry
forums and discussions can provide valuable insights into upcoming regulatory changes, enabling companies to adapt their LoE strategies in a timely and informed manner, especially about their competitons behaviour to anticipate their Go-to-Market strategy. As a result, companies must have a comprehensive understanding of the regulatory environment for developing a robust and adaptable LoE strategy that aligns with the evolving regulatory framework.
Leveraging Brand Advantages
The advantages inherent to branded manufacturers present valuable opportunities for these companies to navigate the complexities of the Loss of Exclusivity (LoE) phase and maintain their competitive edge within the market. These include established brand loyalty, efficient supply chains and logistics, robust customer relationships, and extensive networks. Especially, cultivating strong relationships with healthcare providers, key opinion leaders, and patient advocacy groups, as well as procurement bodies and stakeholders, not only fosters brand backing but also facilitates access to valuable market insights and feedback. Leveraging these relationships enables companies to tailor their product offerings, develop targeted promotional campaigns, and establish collaborative partnerships that support continued brand visibility and market relevance throughout the LoE phase. Therefore, to harness these advantages, companies need a well-structured strategy tailored to the unique dynamics of LoE, enabling companies to maximize their market presence, mitigate competitive threats and sustain their brand value beyond the patent expiry stage.
Empowering with Capabilities and Tools
Inadequate capabilities and tools further hinder effective LoE management. Many Life Sciences companies lack the necessary expertise, data analytics capabilities, and resources to navigate LoE successfully. The absence of a well-defined LoE strategy for capabailities and tools can lead to missed opportunities, unfavorable contracts, and the inability to maintain competitive pricing, given the struggle to accurately assess market trends, forecast demand shifts, and identify emerging opportunities for product differentiation and market expansion. By implementing specialized capabilities and tools, companies can empower their ability to make informed strategic decisions, anticipating opportunities for product diversification and innovative portfolio management. Additional benefits of leveraging critical insights from market data, patient behavior, and competitive intelligence are to develop tailored marketing strategies, optimize pricing structures, or establish effective reimbursement models and securing favorable contracts with key stakeholders – including healthcare providers, insurers, and distributors.
Quantifying Impact for Strategic Decision-Making
Only with the right capabiltiies and tools in place, the company is able to underscore the urgency of LoE management by quantifying the risk and potential losses. For example, inadequate LoE planning can lead to revenue drops of up to 90% for certain products. Therefore, a comprehensive analysis of market size, revenue projections, and case studies illustrating revenue drops reinforces the critical need for proactive LoE management strategies. Additionally, quantifying the potential losses associated with patent expiry serves as a powerful motivator for companies to allocate resources and prioritize investments in developing robust LoE strategies. By contextualizing the potential revenue losses within the broader context of the company’s financial performance and market competitiveness, organizations can effectively communicate the urgency and significance of implementing proactive measures to mitigate the adverse effects of LoE and sustain long-term value retention and profitability. In conclusion, a holistic approach to LoE management, encompassing proactive planning, strategic resource allocation, capabilities and tools, brand leverage, regulatory insights, and quantifying impact, is paramount for Life Sciences companies to ensure sustained competitiveness and success in the dynamic post-patent expiration landscape.
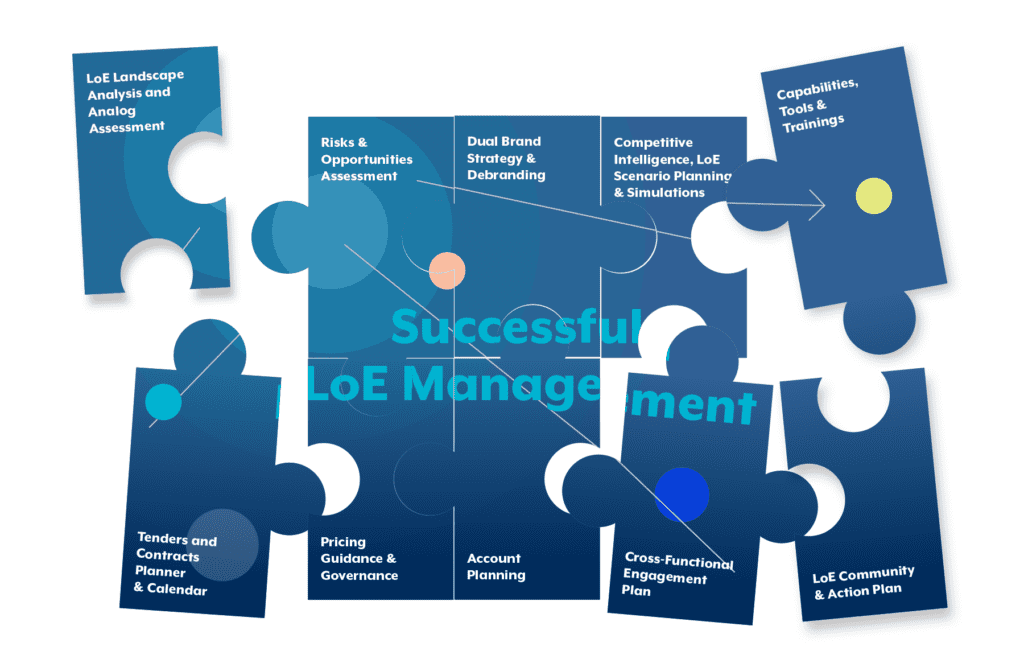
Key Activities for an effective and successful LoE Management
Navigating the intricate landscape of Loss of Exclusivity (LoE) in the Life Sciences industry demands a nuanced approach. Tailoring strategies to individual company needs and their respective asset/product facing LoE, ensures a comprehensive and effective response to the dynamic nature of this phase. The following key 10 activities outlined below cover a spectrum of critical stages, including analysis, strategy development, implementation, and ongoing execution:
1. LoE Landscape Analysis and Analog Assessment
 The foundation of a robust LoE strategy lies in a comprehensive LoE Landscape Analysis. Delving deeply into regulatory pathways, competitive landscapes, and market trends provides invaluable insights to proactively anticipate regulatory hurdles and potential Commercial strategies for oneself, as well as Go-to-Market strategies of the competition. In addition, an Analog Assessment serves as a vital tool by leveraging data-driven insights from past LoE transitions and comparing the current LoE situation to similar cases, gaining valuable guidance and insights that inform the development of comprehensive and adaptive LoE management strategy. Thereby, increasing the organizations chances of minimizing unforeseen risks and maximizing value retention. Moreover, companies should leverage a combination of qualitative and quantitative research methods, including market surveys, data analytics, competitive intelligence, and regulatory trend analysis.
The foundation of a robust LoE strategy lies in a comprehensive LoE Landscape Analysis. Delving deeply into regulatory pathways, competitive landscapes, and market trends provides invaluable insights to proactively anticipate regulatory hurdles and potential Commercial strategies for oneself, as well as Go-to-Market strategies of the competition. In addition, an Analog Assessment serves as a vital tool by leveraging data-driven insights from past LoE transitions and comparing the current LoE situation to similar cases, gaining valuable guidance and insights that inform the development of comprehensive and adaptive LoE management strategy. Thereby, increasing the organizations chances of minimizing unforeseen risks and maximizing value retention. Moreover, companies should leverage a combination of qualitative and quantitative research methods, including market surveys, data analytics, competitive intelligence, and regulatory trend analysis.
By integrating these diverse research methodologies, companies can gain a holistic understanding to draw meaningful comparisons between their current LoE situation and analogous cases in the industry. This strategic approach of LoE Landscape Analysis and Analog Assessment empowers companies to identify niche market segments, potential partnerships, and competitive differentiators, as well as learn from the experiences of others, avoid common pitfalls, and replicate successful approaches to maximize the efficacy of their own LoE management strategies. As a result, this enables companies to position their products effectively to mitigate the impact of generic competition and sustain their market share and value retention during and after the LoE phase.
2. Risks & Opportunities Assessment
 Conducting a wide-ranging Risks & Opportunities Assessment is integral to the successful management of Loss of Exclusivity (LoE). By systematically identifying potential risks and opportunities associated throughout the LoE phase, companies can develop proactive mitigation strategies that enable them to navigate uncertainties with confidence, minimize operational disruptions, and capitalize on emerging opportunities that sustain their market position and retain value and maximize profitability.
Conducting a wide-ranging Risks & Opportunities Assessment is integral to the successful management of Loss of Exclusivity (LoE). By systematically identifying potential risks and opportunities associated throughout the LoE phase, companies can develop proactive mitigation strategies that enable them to navigate uncertainties with confidence, minimize operational disruptions, and capitalize on emerging opportunities that sustain their market position and retain value and maximize profitability.
3. Dual Brand Strategy & Debranding
 A Dual Brand Strategy can serve as a proactive approach for Life Sciences companies to sustain their market share and competitiveness during the Loss of Exclusivity (LoE) phase. By effectively engaging with internal and external stakeholders, companies can develop tailored strategies that leverage dual branding initiatives, allowing them to strategically position their products in the market and maintain a strong market presence despite the challenges posed by generic competition and patent expirations. Therefore, the implementation of a Dual Brand Strategy involves the simultaneous promotion of both the original branded product and its generic counterpart. By highlighting the unique value propositions, superior quality, and distinct attributes of the original brand, companies can continue to cater to customers who prioritize brand loyalty and perceive added value in the branded product. At the same time, effectively positioning the generic alternative as a cost-effective and accessible option enables companies to capture a broader market segment, catering to price-conscious consumers and maintaining market share even in the face of generic competition.
A Dual Brand Strategy can serve as a proactive approach for Life Sciences companies to sustain their market share and competitiveness during the Loss of Exclusivity (LoE) phase. By effectively engaging with internal and external stakeholders, companies can develop tailored strategies that leverage dual branding initiatives, allowing them to strategically position their products in the market and maintain a strong market presence despite the challenges posed by generic competition and patent expirations. Therefore, the implementation of a Dual Brand Strategy involves the simultaneous promotion of both the original branded product and its generic counterpart. By highlighting the unique value propositions, superior quality, and distinct attributes of the original brand, companies can continue to cater to customers who prioritize brand loyalty and perceive added value in the branded product. At the same time, effectively positioning the generic alternative as a cost-effective and accessible option enables companies to capture a broader market segment, catering to price-conscious consumers and maintaining market share even in the face of generic competition.
In instances where the debranding of products becomes necessary, ensuring a seamless transition is paramount to minimize customer confusion and maintain trust. Clear communication strategies, educational campaigns, and transparent messaging are essential for guiding customers through the debranding process, explaining the rationale behind the transition, and emphasizing the continued availability of quality healthcare solutions despite the change in branding. By prioritizing customer education and transparency, companies can navigate the debranding process effectively, mitigate any potential negative impacts on customer perception, and maintain long-term trust and loyalty within their customer base.
However, bear in mind that this strategy may not be applicable nor feasible for all markets and in contrary, based on our experience, only makes commercial sense
on a country-by-country level decision which needs to
be assessed based on the product, its brand, and
market environment.
4. Competitive Intelligence, LoE Scenario Planning & Simulations
 The proactive monitoring of competitors’ actions and market trends is foundational for maintaining a competitive edge during patent expiry. The process of capturing Competitive Intelligence entails employing sophisticated research methodologies, including in-depth market analysis, comprehensive competitor strategies & profiling, product pipelines, pricing models, market positioning and vigilant trend monitoring. All these valuable insights should be leveraged for LoE Scenario Planning & Simuations. Therefore, by developing a comprehensive framework that encompasses best-case, worst-case, and moderate-case scenarios, companies can effectively anticipate the potential challenges, opportunities, and risks associated with LoE, allowing them to devise proactive strategies and contingency plans tailored to each specific scenario. As a result, conducting such LoE simulations facilitates the the proactive identification of potential risk factors and allows to develop mitigation strategies, to optimize resource allocation, streamline operational efficiencies, and implement agile decision-making processes that enable them to adapt to changing market conditions and sustain their competitive edge amidst industry fluctuations and competitive pressures. Additionally, it fosters a culture of strategic foresight which is a critical factor in terms of change management within the organization and helps to maintain trust and confidence.
The proactive monitoring of competitors’ actions and market trends is foundational for maintaining a competitive edge during patent expiry. The process of capturing Competitive Intelligence entails employing sophisticated research methodologies, including in-depth market analysis, comprehensive competitor strategies & profiling, product pipelines, pricing models, market positioning and vigilant trend monitoring. All these valuable insights should be leveraged for LoE Scenario Planning & Simuations. Therefore, by developing a comprehensive framework that encompasses best-case, worst-case, and moderate-case scenarios, companies can effectively anticipate the potential challenges, opportunities, and risks associated with LoE, allowing them to devise proactive strategies and contingency plans tailored to each specific scenario. As a result, conducting such LoE simulations facilitates the the proactive identification of potential risk factors and allows to develop mitigation strategies, to optimize resource allocation, streamline operational efficiencies, and implement agile decision-making processes that enable them to adapt to changing market conditions and sustain their competitive edge amidst industry fluctuations and competitive pressures. Additionally, it fosters a culture of strategic foresight which is a critical factor in terms of change management within the organization and helps to maintain trust and confidence.
5. Capabilities, Tools & Trainings
 Establishing a comprehensive toolkit equipped with the necessary capabilities and tools is essential for Life Sciences companies to streamline the planning and execution of Loss of Exclusivity (LoE) strategies across global and local teams. Therefore, building capabilities within the organization includes providing specialized training programs, workshops, and educational resources that enhance the understanding of LoE dynamics, regulatory compliance, market analysis, and competitive intelligence. By nurturing a workforce with the requisite capabilities, companies can empower their teams to make informed decisions, drive innovation, and adapt to evolving market conditions, ensuring the effective execution of LoE strategies across various regions and markets, whilst building confidence in the organization. Furthermore, implementing advanced tools tailored to LoE planning and execution is integral to optimizing operational efficiency and facilitating seamless collaboration among global and local teams. These tools may include sophisticated data analytics platforms, project management software, and communication tools that enable real-time information sharing, data-driven decision-making, and cross-functional collaboration. By integrating these tools into the organizational workflow, companies can streamline data management, enhance communication channels, and facilitate efficient coordination among teams, thereby ensuring a synchronized approach to LoE planning and execution across diverse markets.
Establishing a comprehensive toolkit equipped with the necessary capabilities and tools is essential for Life Sciences companies to streamline the planning and execution of Loss of Exclusivity (LoE) strategies across global and local teams. Therefore, building capabilities within the organization includes providing specialized training programs, workshops, and educational resources that enhance the understanding of LoE dynamics, regulatory compliance, market analysis, and competitive intelligence. By nurturing a workforce with the requisite capabilities, companies can empower their teams to make informed decisions, drive innovation, and adapt to evolving market conditions, ensuring the effective execution of LoE strategies across various regions and markets, whilst building confidence in the organization. Furthermore, implementing advanced tools tailored to LoE planning and execution is integral to optimizing operational efficiency and facilitating seamless collaboration among global and local teams. These tools may include sophisticated data analytics platforms, project management software, and communication tools that enable real-time information sharing, data-driven decision-making, and cross-functional collaboration. By integrating these tools into the organizational workflow, companies can streamline data management, enhance communication channels, and facilitate efficient coordination among teams, thereby ensuring a synchronized approach to LoE planning and execution across diverse markets.
6. Tenders and Contracts Planner & Calendar
 Optimize your tendering and contracting processes to secure favorable agreements during the LoE period and beyond. Ideally, companies can leverage existing contracting and tendering systems which they have implemented previously and simply need to enhance specific functionalities. Otherwise, companies should explore existing solutions and consider an implementation to support these processes and to develop a comprehensive Tender and Contract Calendar. By organizing and categorizing tender deadlines, contract renewals, and submission timelines, companies can proactively plan their tender submissions, allocate resources effectively, and prioritize strategic segmentation and targeting that maximize their chances of securing positive agreements during the LoE period. This proactive planning approach minimizes the risk of missing critical opportunities, enhances the company’s competitive positioning, and ensures that tender submissions are well-prepared, strategic, and tailored to the specific requirements of each client and market segment.
Optimize your tendering and contracting processes to secure favorable agreements during the LoE period and beyond. Ideally, companies can leverage existing contracting and tendering systems which they have implemented previously and simply need to enhance specific functionalities. Otherwise, companies should explore existing solutions and consider an implementation to support these processes and to develop a comprehensive Tender and Contract Calendar. By organizing and categorizing tender deadlines, contract renewals, and submission timelines, companies can proactively plan their tender submissions, allocate resources effectively, and prioritize strategic segmentation and targeting that maximize their chances of securing positive agreements during the LoE period. This proactive planning approach minimizes the risk of missing critical opportunities, enhances the company’s competitive positioning, and ensures that tender submissions are well-prepared, strategic, and tailored to the specific requirements of each client and market segment.
7. Pricing Guidance & Governance
 Establishing a comprehensive Pricing Guidance and Governance framework is essential for Life Sciences companies to maintain profitability and competitiveness while navigating the complexities of the patent expriy phase. By developing a structured pricing strategy and governance process, companies can anticipate market dynamics, ensure flexible adjustments, and strike the right balance between competitive pricing and revenue preservation, while enabling swift and efficient decision-making with the appropriate approval flow. Therefore, building a pricing guidance framework involves leveraging market research, competitive analysis, and customer insights to develop a well-defined pricing strategy that aligns with the company’s overall business objectives and market positioning. By considering factors such as product differentiation, value propositions, and customer affordability, companies can establish pricing guidelines that optimize profitability, sustain market competitiveness, and mitigate the impact of generic competition and market fluctuations during the LoE phase. Furthermore, by establishing clear decision-making protocols, approval workflows, and cross-functional communication channels, companies can streamline the pricing governance process and enable efficient collaboration among key stakeholders. Ultimately, facilitating fast and flexible decision-making within the pricing governance framework is crucial for responding to market changes, customer demands, and competitive pressures during the LoE phase. This can be achieved by enterprise Price Management & Approvals tools, as well as the LoE Scenario Planning & Simulations,
Establishing a comprehensive Pricing Guidance and Governance framework is essential for Life Sciences companies to maintain profitability and competitiveness while navigating the complexities of the patent expriy phase. By developing a structured pricing strategy and governance process, companies can anticipate market dynamics, ensure flexible adjustments, and strike the right balance between competitive pricing and revenue preservation, while enabling swift and efficient decision-making with the appropriate approval flow. Therefore, building a pricing guidance framework involves leveraging market research, competitive analysis, and customer insights to develop a well-defined pricing strategy that aligns with the company’s overall business objectives and market positioning. By considering factors such as product differentiation, value propositions, and customer affordability, companies can establish pricing guidelines that optimize profitability, sustain market competitiveness, and mitigate the impact of generic competition and market fluctuations during the LoE phase. Furthermore, by establishing clear decision-making protocols, approval workflows, and cross-functional communication channels, companies can streamline the pricing governance process and enable efficient collaboration among key stakeholders. Ultimately, facilitating fast and flexible decision-making within the pricing governance framework is crucial for responding to market changes, customer demands, and competitive pressures during the LoE phase. This can be achieved by enterprise Price Management & Approvals tools, as well as the LoE Scenario Planning & Simulations,
to anticipate pricing adjustments, capitalize on emerging market opportunities, and adapting pricing strategies in real time to maintain a competitive edge and maximize revenue potential throughout the LoE phase and beyond.
8. Account Planning
 Account Planning plays a pivotal role in retaining and growing customer relationships during the critical phase of Loss of Exclusivity (LoE). Conducting in-depth account analyses, customer segmentation, and personalized engagement initiatives, allows companies to proactively address customer concerns, provide transparent communication, and offer value-added services that go beyond product offerings. As such, companies can develop tailored and account-specific strategies, to ensure that their valued customers remain loyal, engaged, and supportive throughout the entire LoE phase, fostering long-term relationships that extend beyond the transitional period and sustain the company’s market position and revenue streams.
Account Planning plays a pivotal role in retaining and growing customer relationships during the critical phase of Loss of Exclusivity (LoE). Conducting in-depth account analyses, customer segmentation, and personalized engagement initiatives, allows companies to proactively address customer concerns, provide transparent communication, and offer value-added services that go beyond product offerings. As such, companies can develop tailored and account-specific strategies, to ensure that their valued customers remain loyal, engaged, and supportive throughout the entire LoE phase, fostering long-term relationships that extend beyond the transitional period and sustain the company’s market position and revenue streams.
9. Cross-Functional Engagement Plan
 Establishing a comprehensive Cross-Functional Engagement Plan is essential for fostering collaboration, communication, and alignment across various functions within the organization. Therefore, by promoting such culture of transparency and open dialogue, companies can break down silos, encourage interdisciplinary collaboration, and leverage the diverse expertise and perspectives of each team to develop wide-ranging and effective solutions. However, this requires the establishment of clear communication channels, defined roles and responsibilities, and structured cross-functional meetings and workshops that promote active participation and input from all teams involved in the LoE management process. By encouraging a culture of inclusivity and mutual respect, companies can ensure that every team is empowered to contribute their unique insights, expertise, and perspectives, raising a sense of ownership and shared commitment to achieving the organization’s LoE goals.
Establishing a comprehensive Cross-Functional Engagement Plan is essential for fostering collaboration, communication, and alignment across various functions within the organization. Therefore, by promoting such culture of transparency and open dialogue, companies can break down silos, encourage interdisciplinary collaboration, and leverage the diverse expertise and perspectives of each team to develop wide-ranging and effective solutions. However, this requires the establishment of clear communication channels, defined roles and responsibilities, and structured cross-functional meetings and workshops that promote active participation and input from all teams involved in the LoE management process. By encouraging a culture of inclusivity and mutual respect, companies can ensure that every team is empowered to contribute their unique insights, expertise, and perspectives, raising a sense of ownership and shared commitment to achieving the organization’s LoE goals.
10. LoE Community & Action Plan
 Setting up an inclusive LoE Community and implementing a comprehensive Action Plan is essential for fostering collaboration, knowledge sharing, and collective problem-solving within the organization. By connecting with country peers, sharing insights, and collaborating on collective actions, companies can address common LoE challenges, promote cross-market collaboration, and leverage the collective expertise and experiences of the internal community to develop effective and tailored solutions that drive sustained success and growth within the dynamic LoE landscape. Furthermore, archetyping similar markets within the LoE Community enables companies to tailor and accelerate the learning and collaboration for specific LoE activities based on market-specific nuances, regulatory landscapes, and customer preferences. By identifying commonalities and differences across similar markets, companies can develop tailored strategies and targeted interventions that address the unique challenges and opportunities presented by each market, promoting a culture of adaptive learning, strategic agility, and cross-market collaboration.
Setting up an inclusive LoE Community and implementing a comprehensive Action Plan is essential for fostering collaboration, knowledge sharing, and collective problem-solving within the organization. By connecting with country peers, sharing insights, and collaborating on collective actions, companies can address common LoE challenges, promote cross-market collaboration, and leverage the collective expertise and experiences of the internal community to develop effective and tailored solutions that drive sustained success and growth within the dynamic LoE landscape. Furthermore, archetyping similar markets within the LoE Community enables companies to tailor and accelerate the learning and collaboration for specific LoE activities based on market-specific nuances, regulatory landscapes, and customer preferences. By identifying commonalities and differences across similar markets, companies can develop tailored strategies and targeted interventions that address the unique challenges and opportunities presented by each market, promoting a culture of adaptive learning, strategic agility, and cross-market collaboration.
Conclusion
In conclusion, pro-active and effective LoE management is crucial for Life Sciences companies to navigate the complex landscape of transitioning from exclusivity to competition. Proactive planning serves as the cornerstone of effective LoE management, allowing companies to anticipate potential challenges, mitigate risks, and capitalize on emerging opportunities that arise during the transition phase. By implementing comprehensive strategies that encompass thorough scenario analyses, cross-functional engagement, and adaptive resource allocation, companies can optimize their operational efficiency and maintain their competitive edge, thereby safeguarding their market share and sustaining their revenue streams amidst the dynamic market shifts and competitive pressures associated with LoE. As such, companies can not only survive LoE transitions but also thrive and unlock opportunities for value retention in a highly competitive market.
To learn more about our experience and how our LoE management services can benefit your organization to enhance your competitive advantage, please contact us to schedule a consultation. Together, we can transform the challenges of LoE into opportunities for value retention and success in the Life Sciences industry.