By Colombe Le Rudulier and Eva Wilson
Introduction
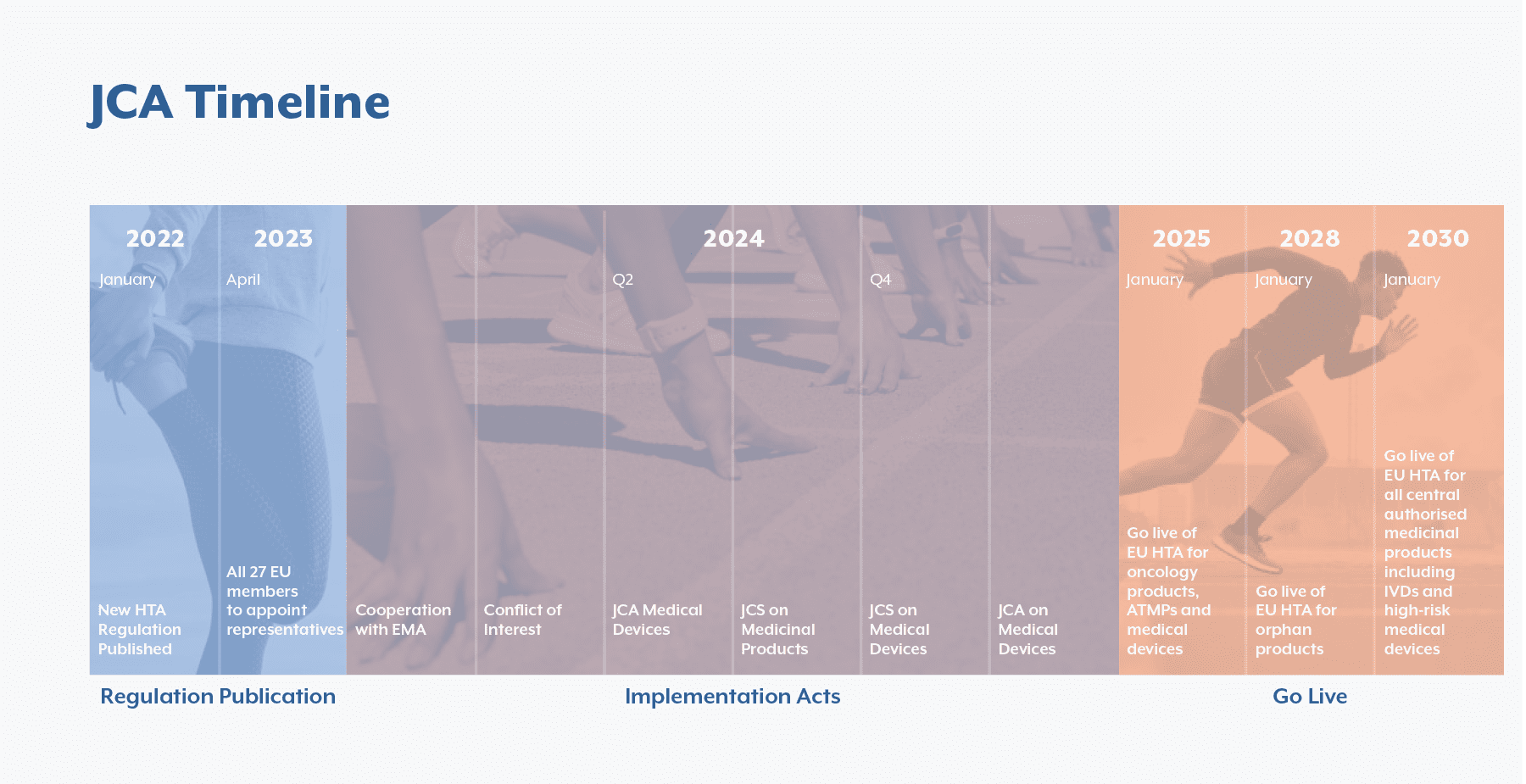
In January 2022 the European Parliament passed the EU regulation 2021/2282. The aim was to standardize clinical assessment of medicines and medical devices across the EU to avoid duplication of work both in-country, as well as at regional European level. This new Health Technology Assessment Regulation (HTAR) will be rolled out in blocks. First, from January 12th 2025, all new oncology products, medical devices and advanced therapy medicinal products (ATMPs)¹ will require to go through the Joint Clinical Assessment (JCA) process. Orphan medicines will follow from January 13th 2028 and lastly all authorized medicinal products excluding generics, biosimilars and vaccines will require to implement the JCA starting January 13th 2030. As we are coming closer to the last stretch before the official launch of the JCA, pharmaceutical and MedTech companies should start preparing for the upcoming race that is JCA implementation.
Ready?
Any runner knows that the pillar to any successful race is training and preparation. To prepare for the new Joint Clinical Assessment (JCA), the Implementing Act (IA)² published by the European Commission in May 2024 is the first step to ensuring a successful start to finish. This document outlines the timelines, procedures and dossier templates involved in the new JCA, which should be used as the basis for planning out the roadmap to January 2025.
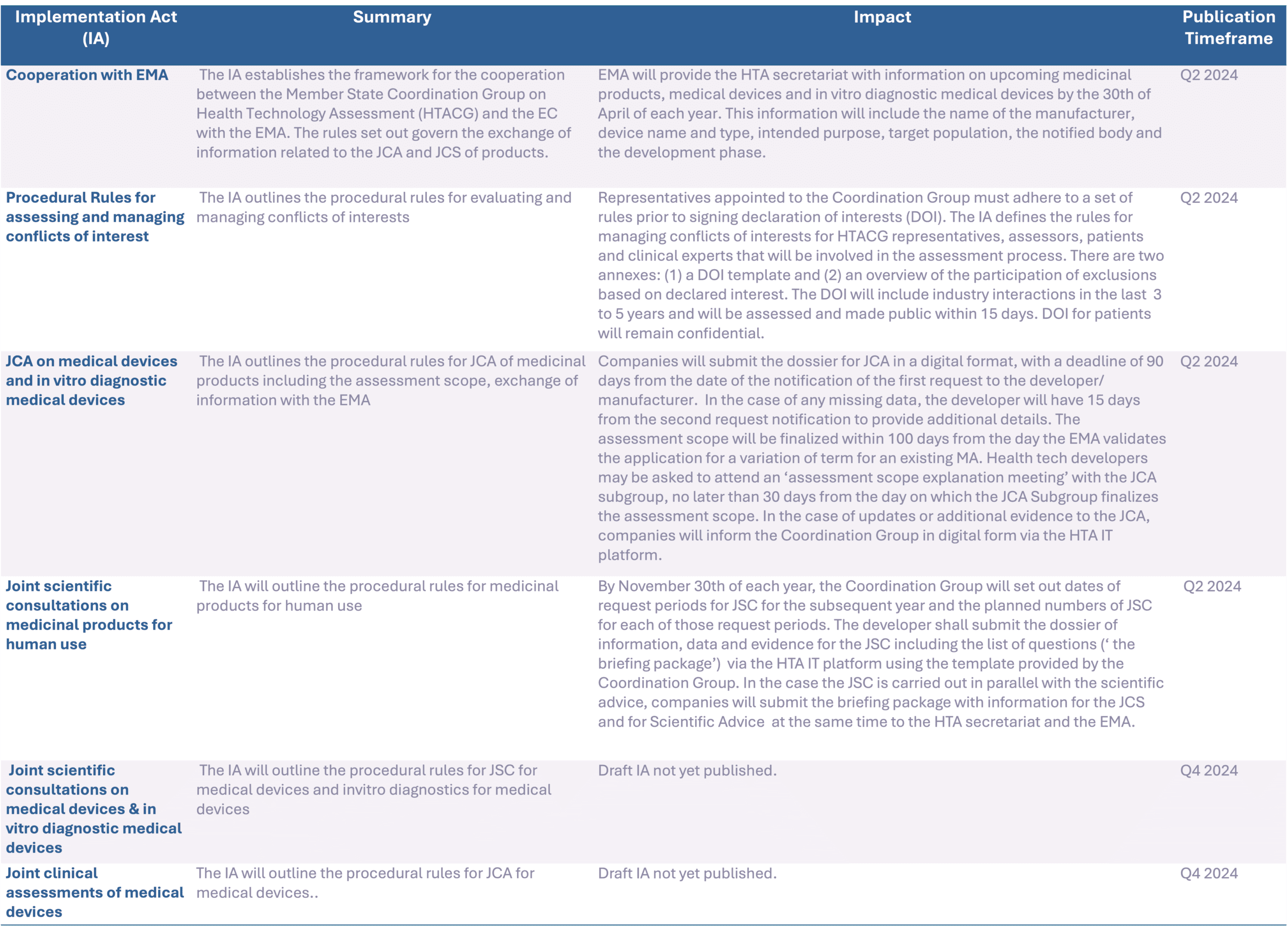
To ensure readiness, the information in the IAs will need to be communicated across teams to manage the changes across various functions. Firstly, companies should have a robust communications plan to ensure the right information is shared internally to the right people. The planning activities, timelines and procedures will need to be communicated to cross-functional teams so that the company is equipped to hit the ground running in 2025. There will be a total of 6 IAs outlined by the HTAR until the regulation enters into force in January 2025, which will focus on information exchange with the EMA, joint scientific consultations for products and conflict of interest management. These IAs will shape the procedures and planning of the JCA so it will be imperative for the industry to stay informed on new requirements and changes that may impact their operations. As of today, 3 out the 6 IAs have been adopted, and 3 are still pending completions.
Secondly, the timelines of implementation lapse from January 12th 2025 for oncology products and ATMPs to January 13th 2030 for vaccines and biosimilars. These lengthy timelines across various product groups will also overlap with other regulatory requirements. Therefore, companies will need to identify the right stakeholders to effectively communicate requirements, activities and timelines to ensure the dossiers are completed and submitted on time, without duplicating work for the Market Access teams.
Finally, the new JCA can be an opportunity for companies to adopt new ways of working by onboarding and preparing teams such as medical affairs and regulatory to collaborate with the Market Access team. Expanding the pool of people being informed on the new JCA process will only benefit companies and further prepare them for the times ahead.
Set …
Now that your team has been prepared to start ahead, setting up the plan based on the IA will help ease teams into the new processes and function required for the Joint Clinical Assessment (JCA). The timelines, dossier template and procedural guidance outlined in the IA will shape the new internal processes for pharmaceutical and MedTech companies.
The change from individual assessments to joint HTAs across Member States will call for a stronger collaboration between local, regional and global teams. A new operating model will need to be established and documented to align with the new JCA functions. The earlier in the race companies begin identifying suitable roles and key expertise for the JCA, the more prepared they will be to meet JCA timelines and yield successful outcomes.
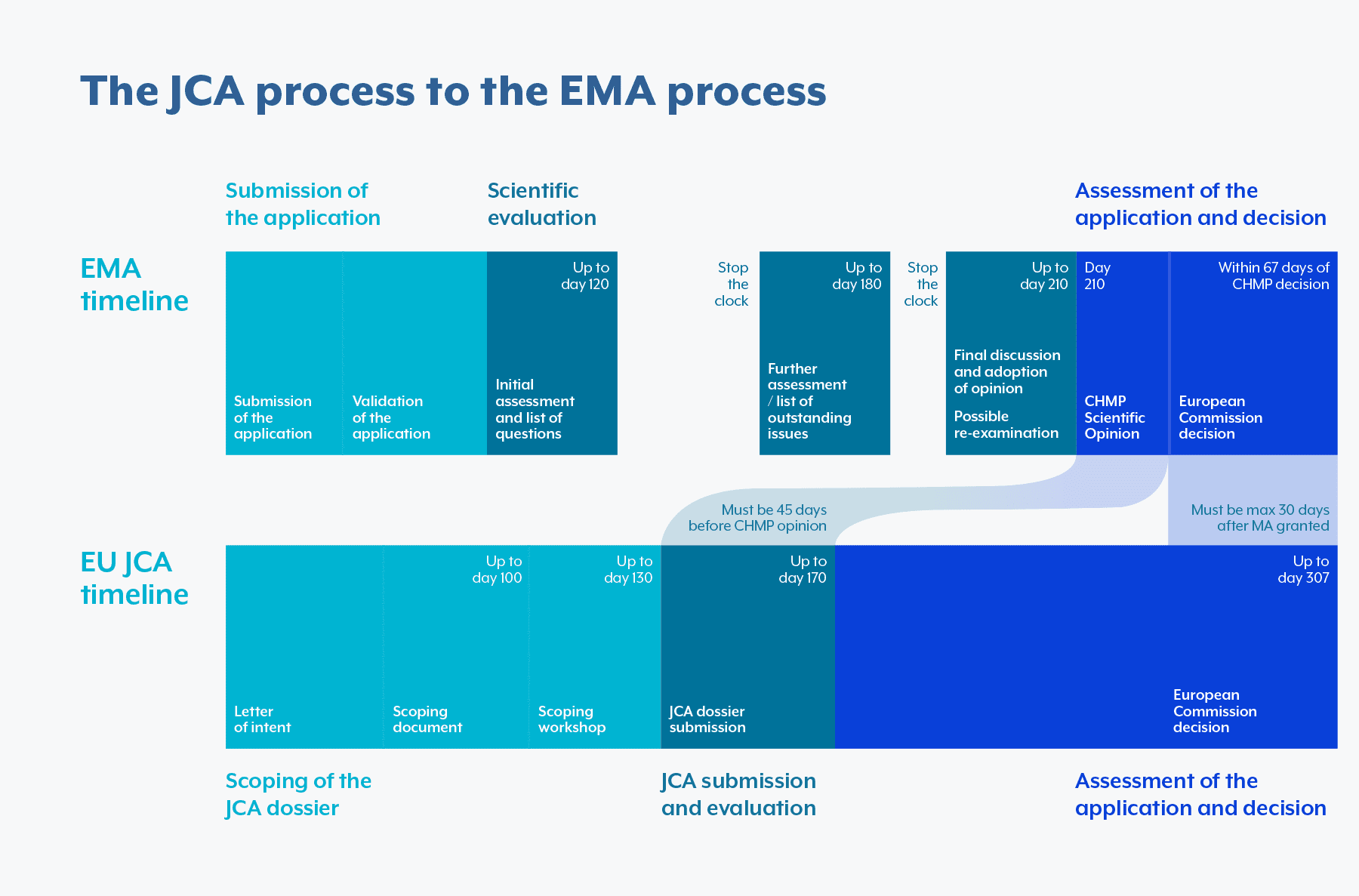
The JCA dossier is set to be submitted within 90 days of the notification request sent by the EC or 60 days for accelerated regulatory procedure, and there is a quick turn-around for responding to data requests post-submission. These tight timelines will overlap with other EMA requests and will require companies to establish new ways of working and internal processes to keep up with the with JCA’s pace.
An approach would be for companies to reassess the interactions between global, regional and local teams, whilst calling for strong collaboration between regulatory teams. A well organized and cross-functional team will help anticipate the (population, intervention, comparator(s), clinical outcome) PICOs anticipated, manage regulatory timelines and reduce duplicate work across all levels. Local teams should focus on the activities that can be done at a local level and provide expertise to their regional and global counterparts.
GO!
The race is nearly about to start; you are now at the starting line. First, it is essential to find the correct starting block, by determining which of the three groups your pipeline belongs to. To that extent, you need to identify the drugs in your pipeline that could be impacted by the HTAR.
The main regulation modification of the HTAR is the preparation and submission of a Joint Clinical Assessment (JCA). Each member state will be able to select PICOs (population, intervention, comparator(s), clinical outcome), which combined will form a PICO framework. The manufacturer will only have 90 days to produce a JCA dossier, adhering to the selected PICO framework. Subsequently, you must ensure that there is ample time to prepare your initial case and dossier, including all safety, clinical, and epidemiological data.
Based on the anticipated needs and potential complications in your request, several steps can be taken to improve your success. In preparation to any big race, it is highly recommended to join a run club. Equivalently, engaging locally with the different Health Technologies Assessment (HTA) bodies and working collaboratively can help anticipate and mitigate potential issues during the JCA. The level of readiness for the HTAR varies greatly between the different European countries. Depending on their involvement in drafting JCA and EUnetHTA activities, some HTA bodies might be better positioned for the HTAR requirements. Furthermore, due to the regulation process, existing ambiguities, the national bodies might implement and use the JCA data differently. Creating a relationship with the member states HTA bodies, can equip manufacturers to better ensure compliance of the HTAR.
Additionally, Pharmaceutical companies have the option to undertake parallel scientific consultation with EMA and HTA bodies. While it is not mandatory, it can help ensure that the preparation and execution of the JCA align with the expectations of the different selected HTAs. This consultation and its results can help identify and generate optimal and robust clinical and pre-clinical evidence. Furthermore, for complex cases with for example multiple indications, warming up by anticipating PICOs might greatly alleviate the preparation. Running a PICO simulation exercise, will help you anticipate and maximise the 90 days session to prepare your JCA dossier.
Conclusion
You are now in the last stretch before the start of the regulatory race imposed by the HTAR. The HTAR requires a meticulous preparation, and a proactive approach. By clearly defining the roles and responsibilities at both the global and local levels, and ensuring that all relevant teams are ready, pharmaceutical companies can optimize their chances of crossing the finish line successfully. Staying on track with the latest guidelines, fostering strong relationships with HTA bodies, and conducting PICO simulation exercise are essential steps to pace yourself, mitigate risks, and enhance outcomes.
Just like in a marathon, early preparation and consistent effort are key to managing the complexities of the Joint Clinical Assessment (JCA) process. However, it is likely the regulation will continue evolving with the first completed cases and the different HTA bodies become more acquainted with the process. Staying adaptable and maintaining open communication with regulatory bodies will be crucial for keeping pace with any changes and ensuring ongoing compliance and success.
Sources
[1] ATMPs can be divided into 3 categories: gene therapy medicines, somatic-cell therapy medicines, tissue-engineered, medicines and combined form of the three.
[2] https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=PI_COM:C(2024)3320