By Lindsey Kinard and Raquel Santos
Digital Therapeutics — A New Era transforming Healthcare
In recent years, the Pharmaceutical Industry has seen the advent of a new era in patient treatment — Digital Therapeutics, transforming and closing a gap within healthcare. Digital Therapeutics, also referred to as DTx, are software-based, evidence-driven healthcare offerings that aid in the treatment, monitoring, or prevention of medical conditions. Seeking to improve patient treatment accessibility and outcomes, these technologies are leveraging advancements in medical science, data analytics, artificial intelligence and consumer technology to augment, or in some cases entirely replace, traditional courses of treatment for a variety of conditions.
To date, these technologies have been produced in a variety of forms — including wearable devices, mobile apps and telemedicine. It’s common within this space for one or more of these forms to be used in conjunction with one another, or in conjunction with traditional treatment plans, to help improve overall patient treatment outcomes.
Throughout this article, we’ll explore the world of Digital Therapeutics. We’ll review current and future Market Insights, discuss approval processes throughout the world, touch on success stories improving the lives of patients and highlight the challenges that the pharmaceutical industry may face when introducing a DTx and how to create successful business strategies.
Digital Therapeutics performance now and in the future
You’ve probably heard the concept that technology doubles itself every few years — this is a layman’s take on Moore’s law, named for the founder of Intel, who observed that roughly every two years the number of transistors on an integrated circuit would double while the relative costs of producing these circuits would remain low.
Anyone who has been alive in the last 20 years can tell you it certainly feels like technology has followed some version of Moore’s law. In half a human lifetime we’ve gone from corded phones in the wall flip phones to smartphones that contain more technology than what got us to the moon.
These exponential leaps in technology are being adopted at an increasing rate in every facet of life — it’s estimated that 54% of the world’s population currently owns a smartphone1 and 84% of healthcare professionals report utilizing at least one form of technology to support their day-to-day operations2.
All of this is to say that the reach of Digital Therapeutics is nearly endless. Technology continues to increase at a rapid rate and no matter what channel you’re looking to serve, from direct-to-consumer to business-to-healthcare provider, the technology that would facilitate DTx is already widely adopted and growing daily.
According to a study conducted by Nova One Advisor, in 2023 the Digital Therapeutics global market was valued at 6.85 Billion USD, with estimates projecting exponential growth surpassing 44.58 Billion USD by 2033. Currently, North America has the lion’s share of DTx revenue, sitting at a staggering 42% revenue share in 2023. Europe follows closely behind at just over 30%, and the Asia/Pacific region comes in third with 21% of the revenue share3.
All of these factors sound promising on paper, but what is truly missing from the market growth conversation is the actual practical side of this; we can project growth all we want, but what are the real areas of use to back it up?
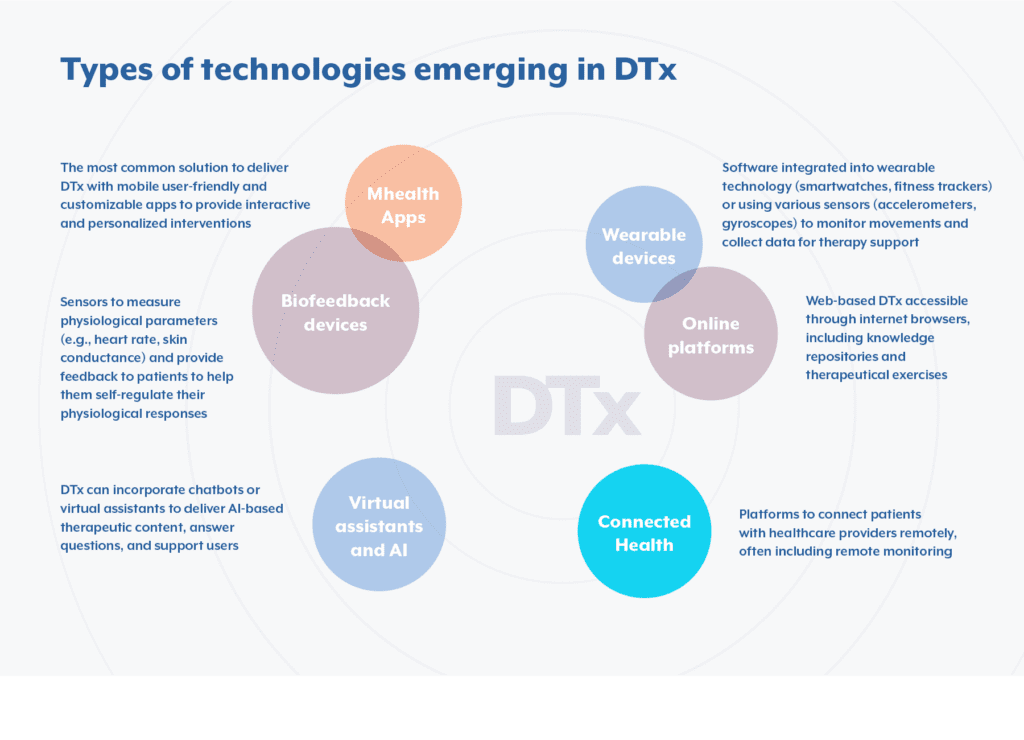
As noted earlier in this article, DTx comes in a variety of forms — and that’s what makes this emerging area ofPharmaceuticals so unique; Digital Therapeutics can fit cleanly into any existing, or potential upcoming, treatments. DTx can utilize a variety of mechanisms to deliver their clinical impact — including, but not limited to, behavioral coaching, biofeedback, gamification cognitive training, neurological/physiologic stimulation, software-determined medication dose modification, and software-directed disease management- the possibilities in augmenting patient treatment are nearly limitless.
- Are patients having a hard time following a dosage regimen? An app can be developed to remind patients to stay compliant with dosages.
- Is there a cancer drug with intense side effects? Create a portal that can be monitored by a patient’s care team for real-time feedback on how dosages are impacting a patient.
- A PTSD (Post-traumatic stress disorder) patient wants an alternative treatment to traditional anti-anxiety medication? A course of treatment with clinically proven breathing exercises can be created within an app to help patients curb symptoms before they get out of control.
Digital Therapeutics — A Case Study
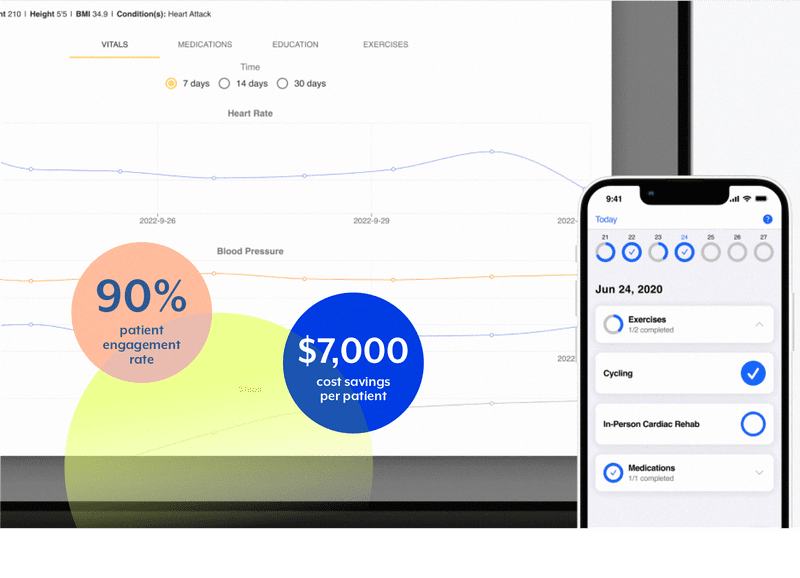
A prime example of a DTx offering that has radically improved the course of patients’ lives can be found at JohnsHopkins University. A team at Johns Hopkins developed a course of treatment for patients who suffered from an Acute Myocardial Infarction. This treatment (dubbed Corrie) involves a multifaceted Digital Therapeutic approach.
Corrie utilizes a smartphone app, smartwatch tracking, and blood pressure monitor to guide patients through their treatment plan. This treatment includes medication reminders, daily exercise goals and tracking, key appointment reminders and the ability for clinicians to review in-app patient health data. A study published in the American Heart Association Journal concluded that Corrie had a 52% reduction in 30-day rehospitalizations. Johns Hopkins additional reports that Corrie has a 90% patient engagement rate and a cost savings of over $7,000 per patient4.
Corrie is a prime example of where Digital Therapeutics shines — it is providing an answer to some of the toughest questions in modern medicine; questions like “how we can improve patient monitoring during critical moments of care?” and “how can we engage patients in their own care?”
Approval process in the USA
Digital Therapeutics — A New Era transforming Healthcare5Approval process in the USAFDA Approval in the United States for DTx is required prior to market use. Currently, the FDA regulates DigitalTherapeutics in the same way that Medical Devices and there are three different classifications to meet different levels of regulatory standards.

- Class I devices are considered “low risk”, and there is minimal review required by the FDA; very few DTx devices currently on the market fall into this category.
- Class II devices are what the majority of DigitalTherapeutic devices currently are and, for theforeseeable future, will be. Class II devices are considered “intermediate risk devices”, and a more thorough (but not exhaustive) review is done by the FDA. Clinical trials may or may not be required, but some form of evidence of its therapeutic effectiveness is required.
- Class III devices are “high risk” or life-sustaining devices; extensive clinical trials and premarket approval are required by the FDA for these devices. Much like Class I, very few DTx devices currently fall into this category.
Regardless of what Class a Digital Therapeutic will fall into, all must be HIPAA compliant, meaning manufactures needto meet specific requirements related to data privacy to protect sensitive health information from disclosure without patient’s consent.
Approval process in Europe
The approval process for DTx in European countries varies depending on the country and the specific product category. No specific regulation exists, however, DTx fall under the Medical Device Regulation (MDR) EU 2017/745framework (5). The classification depends on the intended use and risk associated with the software and similar to theUS, also falls into 3 classes: Class 1 — lower risk, Class IIa and IIb: Moderate-risk devices and Class III: High-risk devices.

Once the classification is determined, manufacturers must undergo a conformity assessment to demonstrate compliance with the MDR. The assessment process involves risk management, evidence supporting clinical efficacy and safety, quality compliance, software validation, including cybersecurity measures and software updates as well as user testing to ensure a user-friendly experience for patients and healthcare professionals.
Once the conformity assessment is complete, the manufacturer can apply the CE mark to the DTx product.The CE mark means the product complies with EU regulations and can be marketed across the EuropeanEconomic Area. For Class I devices, manufacturers self-declare the CE mark after meeting MDR requirements, but for Class IIa, IIb, and III devices, approval from a Notified Body is needed (an organization designated by a member state to assess the conformity of certain products).
Additionally, in Europe, compliance with the General DataProtection Regulation (GDPR) is imperative, as DTx often handles sensitive patient data, and the possibility of integrating with existing healthcare systems is often taken into consideration during the development.
Digital Therapeutics challenges in the pharmaceutical industry
While DTx can be a game-changer for the pharma industry if well-addressed, there are some points that must be considered before starting the development of a DTx to overcome possible challenges. The first step of the long road ahead is the assessment of the stage and digital maturity of the company related to digitalization, including external macro and microenvironment factors.
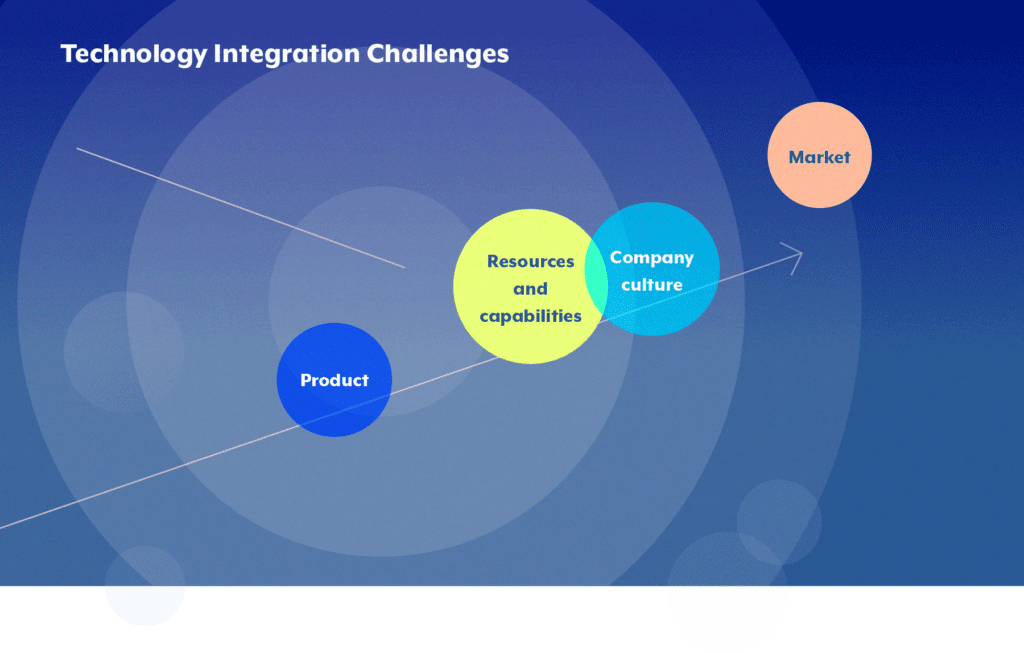
Below we highlight 4 main challenges usually overseen when integrating technology into innovative value-added solutions in the pharmaceutical and medical device industry:
- Market: A market assessment is sometimes neglected leading to process delays or negative outcomes.Understanding the specific key markets and the challenges to face within those markets is key to success. The process of DTx approval, like the drugs or medical devices process, can be lengthy and the reimbursement pathway for this kind of product is sometimes not clearly defined yet, making it difficult to secure reimbursement, which will limit access and adoption by healthcare providers and patients.Communicating effectively with healthcare professionals and patients to adopt and trust digital therapies over traditional treatments is underestimated. An upfront analysis of the market and the recognition of the importance of creating partnerships with the medical community and digital experts is beneficial to generate robust clinical evidence and show long-term benefits, as well as help shape the market for a successful launch.
- Company culture: Pharmaceutical companies are generally built around traditional models of drug development, manufacturing, and distribution and the vision and ambition may be misaligned with the fast pace of digital innovation. DTx requires a shift from product to service-based business models (bundled services), which is a significant change in thinking.Resistance to change or lack of understanding of the digital landscape can impede progress. As a first step, a cultural shift focusing on digital-first should be spread to all employees within the company, with a well-defined adaptation process to digital. Finally, ongoing cross-functional training should be conducted to ensure a smooth transition to a new digital era.
- Resources and capabilities: Digital therapeutics require specialized knowledge in areas like software development, data analytics, and user interface design, which many pharmaceutical companies do not have in-house, leading to difficulties in building, maintaining, and updating digital platforms. Also, building cross-functional teams capable of developing and managing DTx products has not been the priority in the traditional model, and hiring new people can sometimes be difficult to achieve. Also, there is a lack of IT capacities, as DTx products must often be integrated with existing systems to function effectively, which can be a daunting task to achieve and the need to handle large amounts of real-time patient data in a secure and compliant way can be seen as complex and cumbersome. Collaborating with technology firms or third-party digital partners to co-develop DTx solutions may be agood approach to overcome this challenge.
- Product: The DTx product lifecycle completely differs from a traditional pharma drug lifecycle. While a drug requires several years before being approved by a regulatory agency, DTx usually offers a shorter time-to-market because it may require less R&D effort and carry fewer risks. The big challenge starts if a company sees DTx as non-compatible with the current product portfolio, instead of seeing the symbiotic relationship between both. DTx has a disruptive potential when well positioned, and developing bundled product strategies can leverage the potential of both in combination and lead to impressive results, improving patient programs and patient adherence.
How can a pharmaceutical company optimize their revenue adding a DTx to the portfolio?
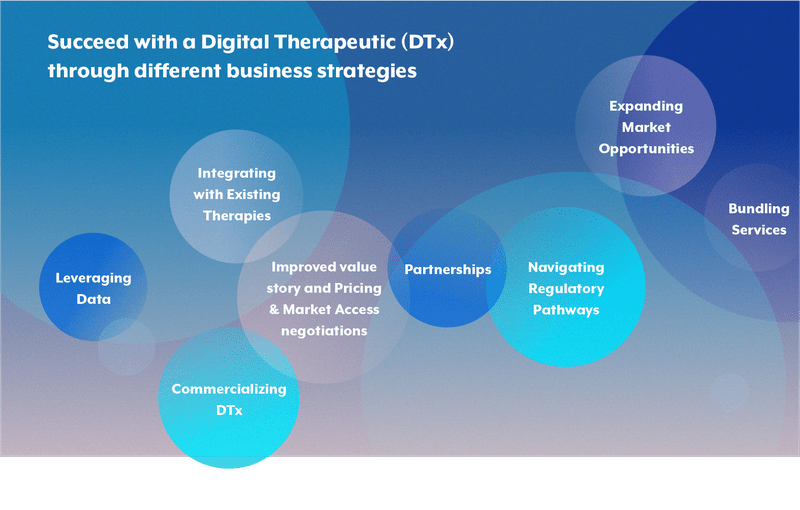
Once the previous challenges are addressed and risks mitigated, companies are in a comfortable position to start recognizing DTx as an added-value product and positioning in the market as differentiators, willing to shape the market and collect the profit. A pharmaceutical company can succeed with a Digital Therapeutic (DTx) through different business strategies:
- Integrating with Existing Therapies: Enhance drug offerings by standing out by offering unique value propositions, such as combining DTx with proprietary drugs or offering specialized solutions for specific conditions to improve patient adherence, leading to better outcomes.
- Improved value story and Pricing & Market Access negotiations: Use real-time monitoring and feedback to optimize treatments, produce positive outcomes, and share cost savings for healthcare systems. Use value-based pricing strategies when negotiating with the authorities.
- Leveraging Data: Generate valuable clinical data to support drug development and regulatory filings and improve patient care. Creating a strategic competitive advantage from artificial intelligence (AI) can also be a possibility to stand out compared to the competition.
- Expanding Market Opportunities: Reach new patient segments and markets by offering digital solutions, especially in underserved areas.
- Partnerships: Collaborate with tech companies and healthcare systems for better adoption and innovation,as well as data usage to improve healthcare.
- Commercializing DTx: Develop standalone DTx products with subscription or licensing models for new revenue streams and predictability. Increased product engagement through updates and upgrades.
- Navigating Regulatory Pathways: even if there is uncertainty related to DTx reimbursement, companies can try efficiently to gain approvals in various markets and develop robust evidence of clinical efficacy and cost-effectiveness to secure reimbursement from insurance companies and government healthcare programs, shaping the market.
- Bundling Services: Offer bundled services that include multiple devices and digital therapies, providing comprehensive care solutions at a competitive price.
DTx are often considered “smart” treatments because they leverage technology, data, and real-time feedback to provide personalized, evidence-based interventions. Differentiation and a unique sell proposition are key for Pharma and MedTech companies to stand out in a crowded market with DTx by offering unique, integrated, and clinically effective solutions that enhance patient outcomes and engagement.
To learn more about our experience and how we can help you with the end-to-end process of setting up digital therapeutics within your portfolio, from its development togo-to-market advisory and including change management initiatives, please contact us to schedule a consultation. Together, we can start transforming the pharma industry by strategically integrating and scaling DTx to the trends of the future, optimizing revenue streams while improving patient care. Starting thinking now is beginning to think ahead for a better and more innovative medicine.
Sources
- https://www.gsma.com/newsroom/press-release/smartphone-owners-are-now-the-global-majority-new-gsma-report-reveals ↩︎
- https://icleanse.com/blog/common-concerns-of-implementing-tablets-in-healthcare ↩︎
- https://www.novaoneadvisor.com/report/us-digital-therapeutics-market ↩︎
- https://www.ahajournals.org/doi/10.1161/CIRCOUTCOMES.121.007741
https://www.corriehealth.com/
https://www.youtube.com/watch?v=0TD96VTf0Xs&t=3429s
https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32017R0745 ↩︎